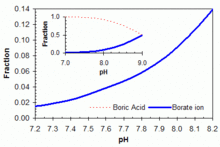
The evaluation only covered the impacts of boric acid in the strip effluent and does not address the other changes in solvents ie the new extractant called MaxCalix more or the new. Sciarra in partial fulfillment of the requirements for the.

Boric Acid Solubility In Ethanol And Acetone Download Table
The boric acid strong liquor high borate concentration is nearly saturated with sodium sulfate.

Boric acid solubility. Mahmoud Abdelaal Thanks for your response. Boric acid has low solubility in water and is used as a source of Boron element and only gives about 2 of Boron WV. Boric acid and borate salts are soluble in water.
Boric acid is sparingly soluble in cold water however fairly soluble in hot water. Solubility of Boric Acid. Boric acid is soluble in the order SO 4 NO 3 and F Cl Br I in common cation solutions.
I need to raise this solubility to get a solution that has Boron 10 View. Boric acid has low solubility in water and is used as a source of Boron element and only gives about 2 of Boron WV. You can either heat it to boiling point to redissolve or you can filter it and this way you will get a 5g 100g solution of boric acid that you.
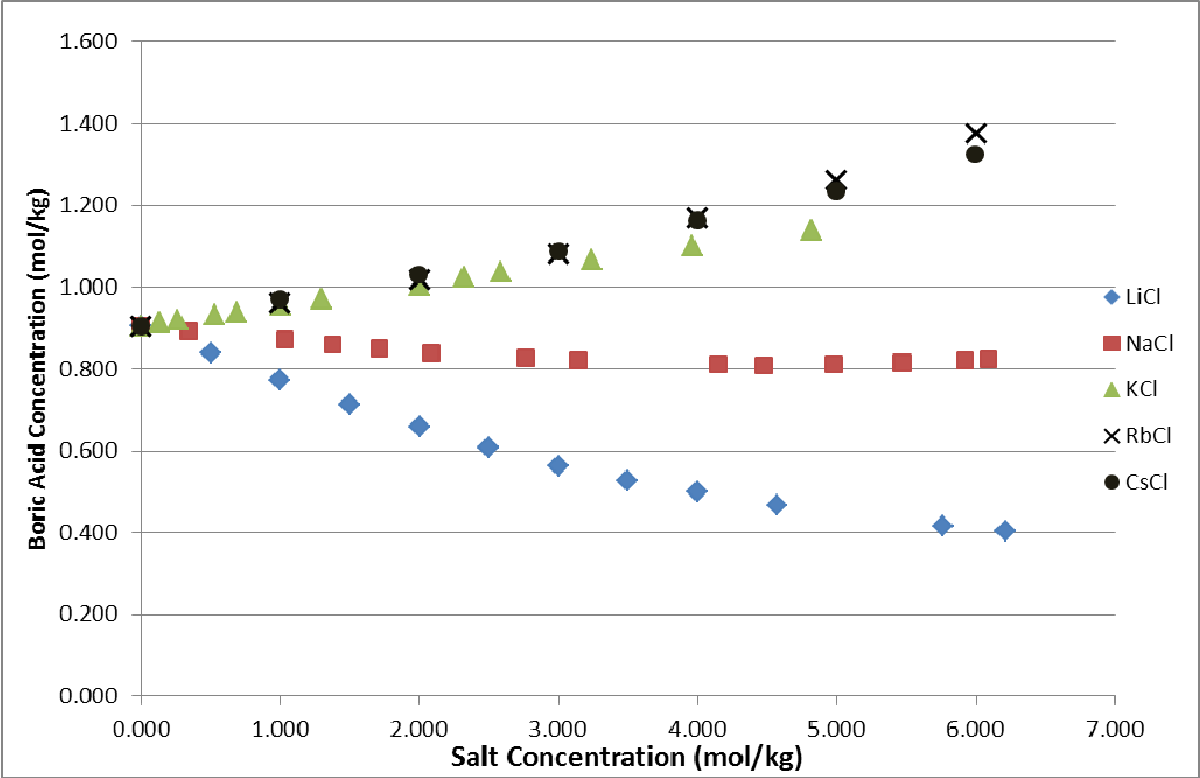
The solubility of boric acid in LiCl NaCl and KCl solutions is not a strong function of temperature and the results can be used over a limited temperature range 535 C. Hatem Ashour Popular answer. Most boron will exist in aqueous solution as boric acid or borate ion.
Boric acid behaves as a weak monobasic acid. When on their own boric acid and borax are only partially soluble in water but when combined they become highly. The use of boric acid has the advantages that it is sufficiently soluble in water to yield the required concentrations it has sufficient chemical and physical stability over the required temperature range and it has a low propensity for.
Understanding what boric acid is will involve knowing its solubility in water. It doesnt act as a proton-donor ie protonic acid however behaves as a Lewis-acid ie it accepts a pair of electrons. However there are potential disadvantages in terms of solubility and hydrolysis reactions and current practice is entirely with boric acid.
Looking at your link the solubility of boric acid at room temperature is around 5g 100g of water. Solutions of boric acid are stable at. To support this effort the impact of using 001M 01M 025M and 05M boric acid in place of 0001M nitric acid was evaluated for impacts on the DWPF facility.
In this report the solubility measurements gravimetric analysis of both 10 B enriched and natural boric acid in heavy warer is described. Boric acid is soluble in boiling water but you need to heat it to above 170C for it to dehydrate. This results in the formation of metaboric acid.
Complete solubility of sodium sulfate is maintained throughout the process by careful control of pH and temperature. The easiest mode of acquisition is in department or hardware stores where it is sold in relatively pure form as roach killer or other pesticides. The solubility of H 3 BO 3 in water is temperature-dependent.
Boric acid is also sold in pharmacies. In common anion salt solutions the order is Cs Rb K Na Li H and Ba Sr Ca Mg. The main problem is that boric acid has a solubility of 11 in ethanol my ethanol is ethanol of 96º the water that it has and produced by the generation of ethylborates give a solubility of 2 so it is actually minor 10.
Presented to the AAAS New York City meeting December 1956. Boric acid has low solubility in water and is used as a source of Boron element and only gives about 2 of Boron WV. Abstract of a dissertation submitted to the Graduate School of the University of Maryland by John J.
Product but with gentle warming it will dissolve to give a clear solution. Boric acid has also been reported to be soluble in alcohol 1 part in 16 and in 85 glycerol solution 1 part in 48 StorageStability A 1 M solution of boric acid in water will have a pH of 35 - 60 at 20 C. It is also slightly soluble in lower alcohols and acetone.
Solubility of ceH3BO3 in various alcohols at 25 C percent by weight. They are removed from soils by leaching and uptake by plants. At a temperature of 25 degrees Celsius the solubility of boric acid in water is 57 grams per litre.
Under standard conditions for temperature and pressure STP boric acid exists as a white crystalline solid that is fairly soluble in water. The strong liquor is filtered at 98 C and boric acid crystallized in two stages using continuous evaporative crystallizers. Boric acid and borate salts are soluble in water.
I need to raise this solubility to get a solution that has Boron 10 Boric. H 3 BO 3 HBO 2 H 2 O. Boric acid is only moderately soluble in water.
Although many solubility data of boric acid in light water are reported previously there are no solubility data of boric acid in heavy water. I need to raise this solubility to get a solution that has Boron 10.

Thermodynamic Modeling Of The Solubility Of Boric Acid In The Systems Boric Acid Lithium Sulfate Water Boric Acid Sodium Sulfate Water And Boric Acid Potassium Sulfate Water At 293 15 313 15 K Sciencedirect

Solubility Of Boric Acid M B As A Function Of Temperature T This Study Download Scientific Diagram

Effect Of The Temperature On Water Solubility And The First Download Table

Effect Of The Temperature On Water Solubility And The First Download Table
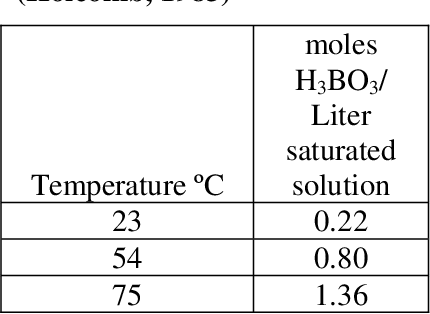
Pdf Literature Review Of Boric Acid Solubility Data Semantic Scholar

Pdf Literature Review Of Boric Acid Solubility Data Semantic Scholar

Boric Acid Solubility In Different Solvents Ullmann S 2006 Download Table

Solubility Study Of Nickel Ferrite In Boric Acid Using A Flow Through Autoclave System Under High Temperature And High Pressure Sciencedirect

Thermodynamic Modeling Of The Solubility Of Boric Acid In The Systems Boric Acid Lithium Sulfate Water Boric Acid Sodium Sulfate Water And Boric Acid Potassium Sulfate Water At 293 15 313 15 K Sciencedirect
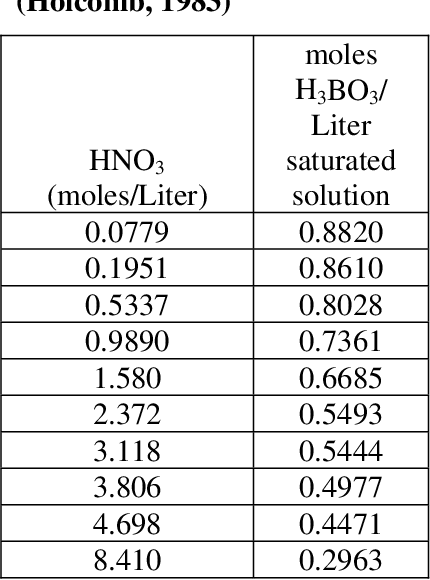
Pdf Literature Review Of Boric Acid Solubility Data Semantic Scholar

Solubility Of Boric Acid And Boron Concentration Depending On The Download Scientific Diagram

Solutions Solubility Rates And Acids Bases Ppt Download
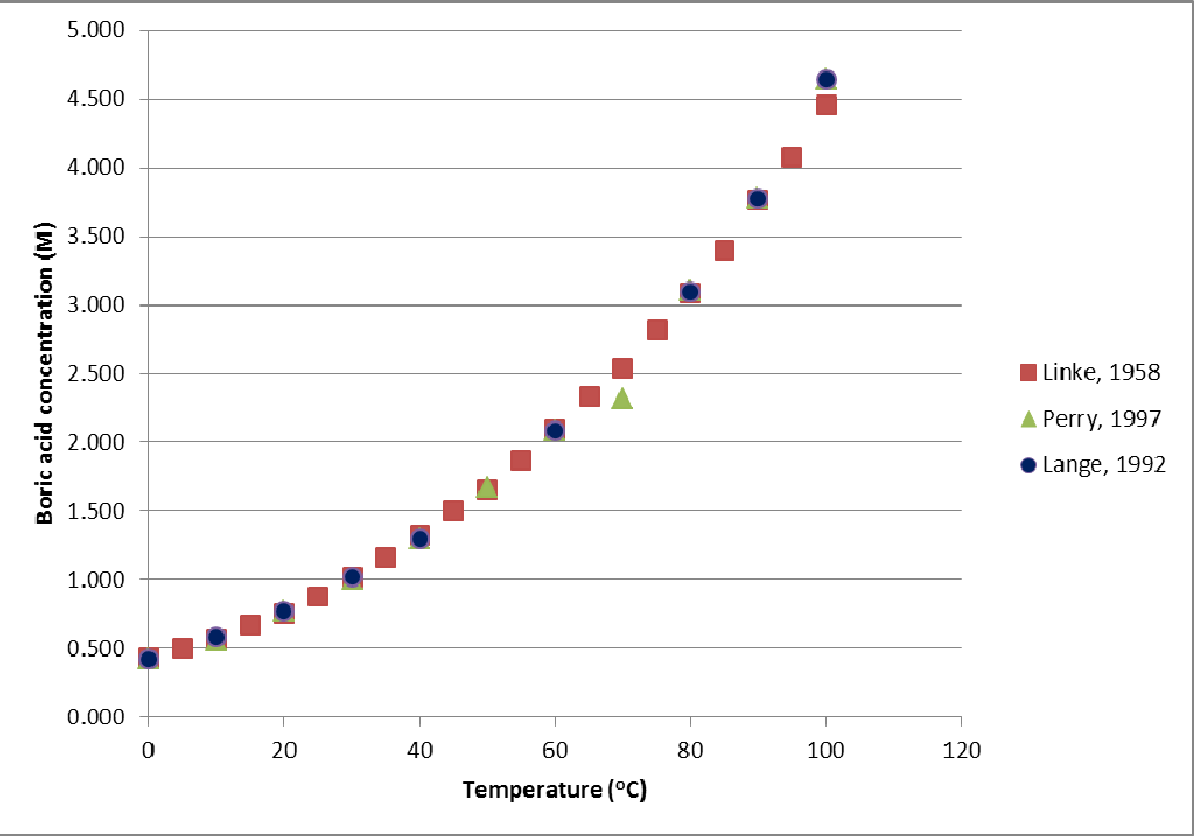
Pdf Literature Review Of Boric Acid Solubility Data Semantic Scholar

Solubility Of Boric Acid M B As A Function Of Sodium Sulfate Molality Download Scientific Diagram
Pdf Literature Review Of Boric Acid Solubility Data Semantic Scholar

Pdf Literature Review Of Boric Acid Solubility Data Semantic Scholar